What is PCOS?
Overview
Polycystic Ovary Syndrome (PCOS) also known as Stein-Leventhal Syndrome (Table 1) is an endocrine disease, which commonly occurred among women at reproductive age (Jayasena and Franks, 2014). It affects 5-20% women in the world (Azziz et al., 2016). Albeit continuous increment in the number of PCOS patients, the main cause of PCOS is still unknown due to the lack of uniform criteria for diagnosis and the heterogeneity of this abnormality (Bargiota and Diamanti-Kandarakis, 2012). However, PCOS is commonly associated with hyperandrogenism, anovulation, amenorrhea, hirsutism, polycystic ovaries, insulin resistance and infertility (Norman et al., 2007). Hormonal imbalance is one of the symptoms that can be recognized among the patients with PCOS. This included an increase in hormone testosterone, estrogen and luteinizing hormone (LH) while a decrease in follicle stimulating hormone (FSH) (De Leo et al., 2016). Besides, PCOS may lead to other severe complications such as cardiovascular disease, diabetic and endometrial cancer (Goodarzi et al., 2011). As of now, no medication to cure this syndrome but it can be managed through a healthy lifestyle such as balanced diet and regular exercises (Jayasena and Franks, 2014). Furthermore, drugs also can be used to manage the symptoms occur in PCOS such as regulation of menstrual cycle, reduction in excessive hair growth and induce ovulation, but most of the drugs may lead to other severe problems (Moghetti et al., 2013; Ehrmann et al., 2016).
Table 1: The preferred name, synonyms, and external IDs of PCOS
| Preferred name | Polycystic Ovary Syndrome |
| Synonyms | Polycystic ovary syndrome [SNOMEDCT_2005_07_31:237055002] |
| Multicystic ovaries [SNOMEDCT_2005_07_31:237792009] | |
| Multicystic ovaries [SNOMEDCT_2005_07_31:190549002] | |
| Polycystic Ovarian disease [NCI2004_11_17:C27086] | |
| Polycystic ovaries [ICD9CM_2006:256.4] | |
| Polycystic ovarian syndrome [ICD10CM_2016:E28.2]* | |
| polycystic ovary [CSP2005:2587-6111] | |
| Stein-Leventhal synd. [SNOMEDCT_2005_07_31:154714009] | |
| PCOS [CSP2005:2587-6155] | |
| Stein-Leventhal synd. [SNOMEDCT_2005_07_31:271199009] | |
| Stein-Leventhal syndrome [MTHICD9_2006:256.4] | |
| Polycystic ovaries (disorder) [SNOMEDCT_2005_07_31:69878008] | |
| Stein-Leventhal syndrome [NCI2004_11_17:C26862] | |
| External IDs | DOID:11612 |
| OMIM:184700 | |
| EFO:0000660 | |
| ICD9CM:256.4 | |
| ICD10CM:E28.2* | |
| MSH:D011085 | |
| SNOMEDCT_2010_1_31:237055002 | |
| SNOMEDCT_2010_1_31:271199009 | |
| UMLS_CUI:C0032460 | |
| NCI:C26862 | |
| Orphanet:3185 |
Source: Ontology Lookup Service (OLS)
*Source: ICD10 version 2016 by World Health Organization (WHO)
Diagnostic Criteria
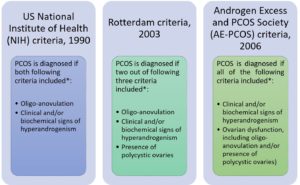
To date, there are three criteria, which are NIH Criteria (Zawadzki and Dunaif, 1992), the Rotterdam Criteria (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004) and AE-PCOS Society Criteria (Azziz et al., 2006) have been used to diagnose PCOS (Figure 1).
*All consensus require the exclusion of other disorders that could mimic PCOS such as congenital adrenal hyperplasia, androgen-secreting tumor, Cushing’s syndrome, 21-hydroxylase-deficient non-classic hyperplasia, androgenic/anabolic drug use or abuse, syndromes of severe insulin resistance, thyroid dysfunction, hyperprolactinemia (Teede et al., 2010).
Figure 1 Three different diagnostic criteria for PCOS
Mechanism/ Pathophysiology
The main cause of PCOS has yet to be elucidated. This might be because of the heterogeneity and complexity of this syndrome (Palomba et al., 2015). However, in order to explain the pathogenesis of this abnormality, several theories or factors including genetics, environment and lifestyle have been proposed (Figure 2).
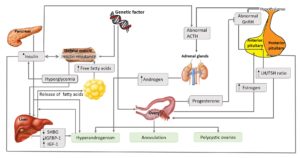
Figure 2: Pathogenesis and pathophysiology of PCOS (Adapted from (20) with minor modification)
According to Figure 2, the abnormal gonadotropin releasing hormone (GnRH) in PCOS women lead to the hypersecretion of luteinizing hormone (LH) prior to ovulation. LH perturbation results in the failure of the oocyte to ovulate. This is the reason women with PCOS have menstrual disturbances. Some women with PCOS may occasionally ovulate while others may not ovulate at all. Such ovulatory problems reduce the fertility of PCOS patients. Besides, anovulation can also cause the estrogen level to remain elevated, which in turn induces the growth of the endometrial lining and suppress the production of follicle stimulating hormone (FSH) (Azziz et al., 2016). Progesterone, which also plays a role in protecting the endometrium and counteracting the effects of estrogen, cannot be produced in anovulatory women (Li et al., 2014). All these factors can result in endometrial hyperplasia, which ultimately increases the risk of developing endometrial cancer in PCOS women (Giudice, 2006).
PCOS is also triggered by high levels of circulating insulin/ hyperinsulinemia/ hyperglycemia, which produces in the β-cells of pancreas (Ehrmann et al., 1995). Environmental or lifestyle factors such as obesity and diet are believed to cause hyperglycemia, which ultimately causes PCOS. Hyperinsulinemia affects anterior pituitary to secrete LH and stimulates ovarian theca to produce ovarian androgen (Weiss et al., 2003) and stimulated hypothalamus to secrete adrenocorticotropic hormone (ACTH) to induce adrenal androgen (Tosi et al., 2011). In the ovary, insulin inhibits production of IGF-binding protein-1 (IGFBP-1) in ovarian granulosa cells, resulting in increased bioavailability of insulin growth factor 1 (IGF-1) and hence, increased ovarian androgen secretion (Poretsky et al., 1996). Insulin may also increase cytochrome P450 17A1 enzyme activity in the ovary, which is an enzyme for biosynthesis of ovarian androgens (Nestler and Jakubowicz, 1996). Besides, insulin inhibits production of IGFBP-1 in liver and increases free IGF-1, which stimulates ovarian androgen production (Lee et al., 1993). Insulin also inhibits production of sex hormone-binding globulin (SHBG) in liver and increases free testosterone, leading to hyperandrogenism (Jayagopal et al., 2003).
Meanwhile, high levels of testosterone, which is one of the subsets of androgenic hormones (Burger, 2002) and alterations of androgenic receptors, such as beta-3 adrenergic receptors and alpha-2 (α2) adrenergic receptors, facilitate catecholamines-induced lipolysis of visceral fat tissues (Pecquery et al., 2015; Xu et al., 1993). This results in high levels of fatty acids in the liver and obesity, ultimately leading to complications such as cardiovascular diseases (Hoffstedt et al., 1996; Lönnqvist et al., 1995).
Symptoms
Symptoms shown by patients with PCOS may vary and possible symptoms that may relate to PCOS are (Azziz et al., 2016; De Leo et al., 2016; Gambineri et al., 2002; Ehrman et al., 1995; Talbott et al., 1995):
- Hirsutism (excessive growth of hair on face, chest, abdomen, and back)
- Hair loss (androgenic alopecia)
- Irregular periods (dysmenorrhea or amenorrhea)
- Polycystic ovaries
- Infertility or reduced fertility
- Obesity
- Acne
However, there is an increased chance that women with PCOS will develop these health problems (Azziz et al., 2016; De Leo et al., 2016; Wild et al., 2000; Dunaif, 1997):
- Diabetes
- High blood pressure and high cholesterol that lead to heart disease
- Irregular periods (dysmenorrhea or amenorrhea)
- Obstructive sleep apnea
- Mood disorders
- Endometrial cancer
Treatments
Up to the present, there is still no cure to treat PCOS. However, medical practitioners usually provide treatments to treat the symptoms shown by patients (Jayasena and Franks, 2014).
- Hormonal birth control (regulates menstrual cycle, reduces testosterone production)
- Anti-androgen medications (reduces abnormal hair growth, acne)
- Diabetes medications such as metformin (lowers insulin levels, regulates menstrual cycle
- Fertility medications (stimulate ovulation to get pregnant)
Besides, medical doctors may advise PCOS patients to change their lifestyle and diet (Glintborg and Andersen, 2017; Jayasena and Franks, 2014). A combination of balanced diet and regular exercise can reduce the patients’ weight. Reduction of weight might help to balance the hormones and improve the menstrual cycle, ovulation and fertility problem.
References
Azziz, R. et. al. (2016) Polycystic ovary syndrome. Nat. Rev. Dis. Prim., 2, 16057.
Azziz, R. et. al. (2006) Position statement: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: An androgen excess society guideline. J. Clin. Endocrinol. Metab., 91, 4237–4245.
Bargiota,A. and Diamanti-Kandrakis,E. (2012) The effects of old, new and emerging medicines on metabolic aberrations in PCOS. Ther. Adv. Endocrinol. Metab., 3, 27–47.
Burger,H.G. (2002) Androgen production in women. Fertil. Steril., 77, S3–S5.
De Leo,V. et al. (2016) Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod. Biol. Endocrinol., 14, 38.
Dunaif,A. (1997) Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis 1. Endocr. Rev., 18, 774–800.
Ehrman,D.A. et al. (1995) Polycystic Ovary Syndrome as a Form of FunctionalOvarian Hyperandrogenism Due to Dysregulation of Androgen Secretion. Endocr. Rev., 16, 322–353.
Ehrman,D.A. et al. (2016) Effects of Metformin on Insulin Secretion, Insulin Action, and Ovarian Steroidogenesis in Women with Polycystic Ovary Syndrome *. 82, 524–530.
Ehrman,D.A. et al. (1995) Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J. Clin. Invest., 96, 520–7.
Gambineri,A. et al. (2002) Obesity and the polycystic ovary syndrome. Int. J. Obes. Relat. Metab. Disord., 26, 883–896.
Giudice,L.C. (2006) Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract. Res. Clin. Endocrinol. Metab., 20, 235–244.
Glintborg,D. and Andersen,M. (2017) MANAGEMENT OF ENDOCRINE DISEASE: Morbidity in polycystic ovary syndrome. Eur. J. Endocrinol., 176, R53–R65.
Goodarzi,M.O. et al. (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol., 7, 219–231.
Hoffstedt,J. et al. (1996) The metabolic syndrome is related to (beta)3-adrenoceptor sensitivity in visceral adipose tissue. Diabetologia, 39, 838–844.
Jayagopal,V. et al. (2003) The Biological Variation of Testosterone and Sex Hormone-Binding Globulin (SHBG) in Polycystic Ovarian Syndrome: Implications for SHBG as a Surrogate Marker of Insulin Resistance. J. Clin. Endocrinol. Metab., 88, 1528–1533.
Jayasena,C.N. and Franks,S. (2014) The management of patients with polycystic ovary syndrome. Nat. Rev. Endocrinol., 10, 624–636.
Lee,P.D.K. et al. (1993) Insulin-like growth factor-binding protein-1 response to insulin during suppression of endogenous insulin secretion. Metabolism, 42, 409–414.
Li,X. et al. (2014) Endometrial progesterone resistance and PCOS. J. Biomed. Sci., 21, 2.
Lönnqvist,F. et al. (1995) A pathogenic role of visceral fat beta 3-adrenoceptors in obesity. J. Clin. Invest., 95, 1109–16.
Moghetti,P. et al. (2013) Divergences in Insulin Resistance Between the Different Phenotypes of the Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab., 98, E628–E637.
Nestler,J.E. and Jakubowicz,D.J. (1996) Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N. Engl. J. Med., 335, 617–23.
Norman,R.J. et al. (2007) Polycystic ovary syndrome. Lancet (London, England), 370, 685–97.
Palomba,S. et al. (2015) Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int. J. Womens. Health, 7, 745–763.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril, 81, 19–25.
Pecquery,R. et al. (2015) Influence of Androgenic Status on the α2/β-Adrenergic Control of Lipolysis in White Fat Cells: Predominant α2-Antilipolytic Response in Testosterone-Treated-Castrated Hamsters. Endocrinology, 122, 2590–2596.
Poretsky,L. et al. (1996) Insulin Receptor Mediates Inhibitory Effect of Insulin, but Not of Insulin-Like Growth Factor (Igf)-I, on Igf Binding Protein 1 (Igfbp-1) Production in Human Granulosa Cells. J. Clin. Endocrinol. Metab., 493–496.
Talbott,E. et al. (1995) Coronary heart disease risk factors in women with polycystic ovary syndrome. Arterioscler. Thromb. Vasc. Biol., 15, 821–6.
Teede,H. et al. (2010) Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med., 8, 41.
Tosi,F. et al. (2011) Insulin enhances ACTH-stimulated androgen and glucocorticoid metabolism in hyperandrogenic women. Eur. J. Endocrinol., 164, 197–203.
Weiss,J.M. et al. (2003) Effects of insulin on luteinizing hormone and prolactin secretion and calcium signaling in female rat pituitary cells. Arch. Gynecol. Obstet., 269, 45–50.
Wild,S. et al. (2000) Cardiovascular disease in women with polycystic ovary syndrome at long‐term follow‐up: a retrospective cohort study. Clin. Endocrinol. (Oxf)., 52, 595–600.
Xu,X. et al. (1993) Postreceptor events involved in the up-regulation of β-adrenergic receptor mediated lipolysis by testosterone in rat white adipocytes. Endocrinology, 132, 1651–1657.
Zawadzki,J.K. and Dunaif,A. (1992) Diagnostic criteria for polycystic ovary syndrome: towards a rational approach.